Random and Systematic Errors
When measuring data unavoidable errors will occur. These errors can be classified into three categories:
- Systematic errors: errors which are due to, for example, unclean samples or uncalibrated measurement devices. Systematic errors often show up as a constant or proportional shift of the measured values. Systematic error effect the accuracy of the mean (sometimes called "trueness")(1) and introduce a bias.
- Random errors: originate from random processes in the measurement device or the sensor (e.g. the thermal noise of a sensor, or the quantization noise). Random errors influence the precision of a measurement.
- Outliers (extreme values): outliers are single values lying far outside the usual or typical measurements. Big outliers can be detected and should be removed.
The concepts of trueness and precision can be easily understood when you think of a dart game. If the player shows a high accuracy of the mean, she will hit - on average - the center of the dart board. However, this is of little use, if the precision is poor. On the other hand, a player having a calm hand may achieve high precision although she constantly hits a spot besides the center.
 | 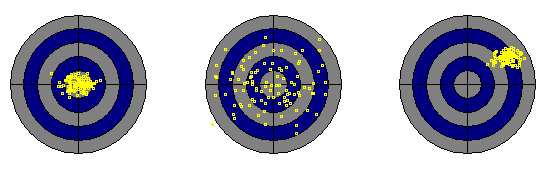 |
| Left: high accuracy of the mean and high precision. Center: high accuracy of the mean at low precision. Right: low accuracy of the mean at high precision. The right example obviously shows a systematic error (bias). Click the image to start the interactive example. |
If we interpret the differences between trueness and precision by statistical terms, it is evident that the trueness of a measurement can be described by the mean (or, in general, by any other measures of location of the distribution of the measured values), and the precision may be described by the standard deviation (or another measure of variation).
Examples of systematic errors
Reference errors:
- Invalid calibrations may result both in additive and multiplicative errors.
- References based on volumes (i.e. volumetric flasks) used at the wrong temperature
- Thermoelements always return the difference between the cold and the warm junction. If the temperature of the cold junction is subjected to a drift, the measured temperature value will change.
- Ageing of sensors may shift a calibration.
Errors of the measurement procedure:
- Chemical detection reactions which do not reach equilibrium (due to slow kinetics).
- Instability of certain species may result in changing signals during measurement.
- Cross sensitivities are, in general, a major problem in complex analytical samples.
- Personal mistakes may be due to oddities of the investigator. Operator and lab performance may be checked by ringtests.
|

 Basic Concepts
Basic Concepts  Precision and Accuracy
Precision and Accuracy  Random and Systematic Errors
Random and Systematic Errors Basic Concepts
Basic Concepts  Precision and Accuracy
Precision and Accuracy  Random and Systematic Errors
Random and Systematic Errors

